Treatment Options
Oocyte Pick-up/Retrieval
ICSI
Embryo Freezing
Frozen Embryo Transfer
Genetic Testing

In vitro Fertilization (IVF)
In Vitro Fertilization is procedure used to treat certain fertility conditions. With In vitro fertilization, the fertilization of an egg takes place outside of the body. A woman’s eggs are removed from her ovaries, placed in a laboratory culture dish and then mixed with sperm. Fertilization takes place in the dish. Once the eggs have been fertilized and embryos have formed, one to two embryos are placed back inside the woman’s uterus. If the embryo implants in the uterus is successful, the woman becomes pregnant. Our IVF clinic in Calgary has helped thousands of couples conceive and become parents.
When is IVF used?
IVF is often considered as a first line treatment for women with diminished ovarian reserve and severe male factor infertility in which case ICSI is used. However, it is also indicated for several other reasons and may be needed when other treatments fail or in cases of unexplained infertility.
What to expect:
When an IVF cycle is the treatment option you and your physician decide is right for you, you can expect the following.
- Ovarian Stimulation – Ovarian stimulation is performed (controlled ovarian hyperstimulation) using injectable hormones (gonadotrophins). Cycle monitoring is done using ultrasound scans. The aim of treatment is to develop an appropriate number of eggs in your ovary. This is done by adjusting the dose of the medication based on the results of ultrasound scans and blood tests. Ovulation is triggered (HCG) and egg retrieval is scheduled 36 hours later.
- Egg Retrieval – Egg retrieval is a surgical procedure performed under conscious sedation in our specially designed operating room at the Oasis Fertility Centre. The retrieval is done using a needle mounted on an ultrasound scan probe. This probe is the same as the one used for cycle monitoring. The eggs obtained are handed to our expert team of embryologists. This procedure takes 20 to 30 minutes.
- Insemination, Fertilization and ICSI – Eggs retrieved are either placed in specialized IVF dishes and mixed with your partners sperm, or in cases of severe male factor infertility the sperm is directly injected into the egg using a procedure known as intracytoplasmic sperm injection (ICSI). The fertilized eggs are then placed in our state-of-the-art incubators for further development. At Oasis Fertility Centre we use real-time time lapse monitoring to assess the health of the embryos which helps us decide which are the best embryos to place in your uterus.
- Embryo Transfer – Fertilized eggs are called embryos. One or two embryos are transferred into the uterus either on day 3 or 5 of the cycle depending on various factors including the number of embryos available, their quality, and other clinical considerations. This is a painless procedure using a narrow catheter inserted under ultrasound scan guidance. The procedure generally takes 15 to 20 minutes. Progesterone, which was started before the egg retrieval, is continued until your pregnancy test. Progesterone may be in the form of either an intravaginal capsule or intramuscular injection.
- Freezing of Embryos – When there are more good quality embryos than are being transferred, you will have the option to freeze these embryos so that can be used at another time to try to achieve a pregnancy. The process used to freeze embryos is called vitrification.
- Pregnancy – A blood pregnancy test is performed approximately 2 weeks following the embryo transfer and is generally repeated within 48 hours to confirm pregnancy. A viability ultrasound scan is performed when you are approximately 6 weeks pregnant. You will then be offered of choice of being referred to your family physician or an obstetrician of your choice.
Complications:
There are some risks associated with the IVF Procedure including infection and bleeding, along with:
Multiple Birth
Multiple Birth is a serious complication of any fertility treatment.
Miscarriage
A miscarriage is defined as the loss of a fetus before 20 weeks of pregnancy. 80% of miscarriages occur within the first 13 weeks of pregnancy. There is a slightly higher chance (approximately 22%) of a miscarriage when undergoing IVF. Women over the age of 35 have a higher (20% to 35%) chance of
having a miscarriage than younger women. Chromosomal abnormality of the fetus is the most common reason why a miscarriage occurs. Women over the age of 35 and men over the age of 40 are more likely to be affected by chromosomal abnormalities than younger couples. If you have had multiple
miscarriages Comprehensive Chromosomal Screening (CCS) should be considered.
Stress
Undergoing any fertility treatment can be physically, emotionally, and financially stressful. We at Oasis Fertility Centre recognize how important it is to alleviate stress as much as possible to improve your fertility treatment outcomes. We have dedicated health care professional that can offer you support and counselling.
Success rates:
Success rates for IVF are very dependent upon the age of the woman.
National Averages representing live birth rates per embryo transfer, with a woman using her own eggs:
Age
Success Rate (%)
Under 35
41
35-37
34
38-40
11
41-42
6
These figures represent the National Average, live birth rates. Per embryo transfer with a woman using her own embryos. Based on 2016 figures.
Oocyte Pick-up/ Retrieval
ICSI
ICSI is a procedure whereby one sperm is injected directly into an egg for fertilization purposes. It is done in conjunction with an IVF treatment. The injection takes place in the lab, after the eggs have been retrieved.
When is it used?
What to expect:
From a patient perspective, ICSI is exactly the same as undergoing a regular cycle of IVF. The difference is that in conventional IVF several sperm are placed in a dish with the eggs to allow fertilization to occur naturally, where as with ICSI, one sperm is injected directly into the egg with the hope of fertilization occurring.
Complications:
The main complications associated with ICSI are actually those that arise from the IVF procedure itself. These include ovarian hyperstimulation, multiple birth, ectopic pregnancy, miscarriage, and stress. Patients often question if there is a higher risk of birth defects when ICSI is used. To date, research shows that the risk of birth defects after ICSI is the same as for babies conceived by IVF without ICSI. However, some studies have suggested that having IVF with or without ICSI might increase the risk for birth defects.
Success Rates:
ICSI greatly improves the odds of fertilization. Approximately 70% to 85% of eggs are fertilized when using the ICSI procedure. This does not mean that all of the eggs will develop into embryos that are suitable for transfer.
Embryo Freezing
It is a well-known fact that women’s biological clock results in decreasing fertility and ovarian reserve as they age. This decline is exaggerated after the age of 35. Egg (oocyte) freezing allows the freedom for women to preserve their fertility while their eggs are in their prime.
When is it used?
There are several reasons why you may choose to freeze your eggs. From a social perspective, you may not yet have found the right partner, are concentrating on establishing your career, wish to continue your education, and want to ensure that when you are ready to have a family, you are able to. Oocyte freezing allows you to have this option.
From a medical perspective, oocyte freezing is often recommended for young women undergoing cancer treatment since chemotherapy and/or pelvic radiation has the potential to affect the ovaries. It should also be considered for any surgery which can impact ovarian function, or for women whose families have a history of premature ovarian failure as a result of a chromosomal abnormality or has a history of premature menopause.
What to expect:
You will be required to have all the same tests as those women undergoing a cycle of IVF (with the exception of the semen analysis).
Once your physician has determined that you are a candidate for egg freezing you will under go the first two steps of the IVF procedure, ovarian stimulation and egg retrieval. Once the eggs have been retrieved they will be examined under a microscope and the mature eggs will be cryopreserved (an ultra rapid cooling technique where the eggs are stored in liquid nitrogen).
Complications:
The complications that can arise from Egg Freezing include infection, bleeding, ovarian hyperstimulation syndrome (OHSS) and stress.
Success Rates:
Egg freezing is a relatively new technology with limited data available on success rates. Pregnancy success rates are dependent upon the age of the woman freezing her eggs and the number of viable eggs frozen.
Frozen Embryo Transfer
Frozen embryos are those embryos that were not used during a fresh IVF cycle and were frozen to use at a later date. For a frozen embryo transfer, the embryos are thawed and transferred back into your prepared uterus. It does not require the ovaries to be stimulated as they are for an IVF cycle, however, it does require the uterine lining be thickened before transfer.
When is it used?
Frozen embryos seem to have an infinite life span. They can be used to expand your family when you are ready, after an unsuccessful fresh cycle if frozen embryos are available or after CCS.
What to expect:
In order for the embryo to implant, the uterus must be ready for implantation. Typically, this means that medications such as the birth control pill, Lupron or Synarel will be prescribed to stop you from ovulating unexpectedly. Once your pituitary has been suppressed you will start to take estrogen to thicken the lining of your uterus. Monitoring through transvaginal ultrasound and blood tests will be necessary to make sure that the uterus is thickening as it should. Once the uterine lining is sufficiently thick, progesterone will be added and the Lupron or Synarel will be stopped. Progesterone matures the lining and makes it receptive to embryo implantation. Your transfer will be scheduled accordingly.
Complications:
As with any transfer, there is always a risk of infection or bleeding. If you become pregnant, risks associated with pregnancy such as an ectopic pregnancy, miscarriage, premature birth or multiple birth can also occur.
Success Rates:
Pregnancy success rates after a frozen embryo transfer are the same as that of a fresh IVF cycle. For women under the age of 35, pregnancy rates can be as high as 60 %.
Pre-Implantation Genetic Screening
Preimplantation Genetic Screening (PGS) refers to a set of techniques used during in vitro fertilization (IVF) to screen embryos for genetic abnormalities before they are transferred to the uterus. PGS aims to increase the chances of a successful pregnancy and reduce the risk of passing on certain genetic conditions or chromosomal abnormalities to the child. Chromosomal abnormalities are a frequent cause of failed implantation, recurrent implantation failure and miscarriage.
There are three main types of PGS:
1. PGT-A (Preimplantation Genetic Testing for Aneuploidy):
This is the frequently performed kind of PGT-A performed, and is used to screen embryos for chromosomal abnormalities, specifically aneuploidy. Aneuploidy refers to the presence of an abnormal number of chromosomes, such as having an extra or missing chromosome. PGT-A helps identify embryos with chromosomal abnormalities, such as Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), or Patau syndrome (trisomy 13). By selecting embryos without these abnormalities, the chances of a successful pregnancy and the birth of a healthy child can be improved.
2. PGT-M (Preimplantation Genetic Testing for Monogenic Disorders):
PGT-M is used to screen embryos for specific single-gene disorders or inherited conditions. It is typically used when one or both parents carry a known genetic mutation that could be passed on to their children such as cystic fibrosis, muscular dystrophy, and other genetically transmitted conditions. PGT-M allows identification of embryos unaffected by the specific genetic disorder being tested. This helps reduce the risk of passing on the genetic condition to future generations.
3. PGT-SR (Preimplantation Genetic Testing for Structural Rearrangements):
PGT-SR is used to screen embryos for structural rearrangements in their chromosomes. Structural rearrangements can include deletions, duplications, inversions, or translocations. PGT-SR is often utilized when one or both parents have a known structural rearrangement in their chromosomes. The aim is to identify embryos that do not carry these rearrangements or have a balanced form of the rearrangement, which reduces the risk of passing on an unbalanced form to the child. The specific testing techniques and methods used may vary depending on the type of PGS being performed.
How is PGT-A performed?
- IVF Procedure: PGT-A is typically performed as part of an IVF cycle.
- Following retrieval of the eggs from the ovary and fertilization, the embryos are allowed to develop for 5 – 7 days. The embryo at this stage is called a blastocyst.
- Biopsy: A small number of cells are carefully removed from each embryo’s outer layer (trophectoderm) using a specialized technique.
- Genetic Analysis: The biopsied cells undergo genetic analysis to determine the chromosomal status of each embryo. Various techniques, such as next generation sequencing or microarray analysis, are used to assess the embryo’s genetic makeup.
- Embryo Selection: Based on the genetic analysis results, the embryos with the correct number of chromosomes are identified for transfer to the uterus and are graded according to their physical appearances.
- Embryo Transfer: The selected embryos are transferred to the uterus, to allow for implantation to occur.
What are the benefits of PGT-A?
Increased Pregnancy Success: PGT-A helps identify embryos with the correct number of chromosomes, which improves the chances of a successful pregnancy (see graph below), and reduces the number of attempts to realizing a live birth.
When is it used?
Frozen embryos seem to have an infinite life span. They can be used to expand your family when you are ready, after an unsuccessful fresh cycle if frozen embryos are available or after CCS.
What to expect:
In order for the embryo to implant, the uterus must be ready for implantation. Typically, this means that medications such as the birth control pill, Lupron or Synarel will be prescribed to stop you from ovulating unexpectedly. Once your pituitary has been suppressed you will start to take estrogen to thicken the lining of your uterus. Monitoring through transvaginal ultrasound and blood tests will be necessary to make sure that the uterus is thickening as it should. Once the uterine lining is sufficiently thick, progesterone will be added and the Lupron or Synarel will be stopped. Progesterone matures the lining and makes it receptive to embryo implantation. Your transfer will be scheduled accordingly.
Complications:
As with any transfer, there is always a risk of infection or bleeding. If you become pregnant, risks associated with pregnancy such as an ectopic pregnancy, miscarriage, premature birth or multiple birth can also occur.
Success Rates:
Pregnancy success rates after a frozen embryo transfer are the same as that of a fresh IVF cycle. For women under the age of 35, pregnancy rates can be as high as 60 %.
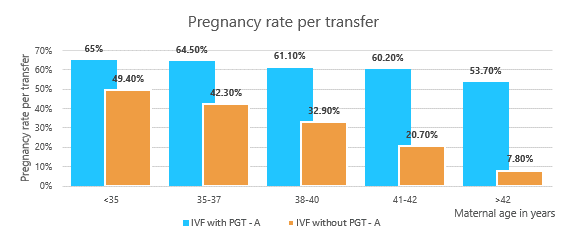
Reduced Risk of Miscarriage: By selecting chromosomally normal embryos, the risk of miscarriage due to aneuploidy is significantly reduced.
Decreased Risk of Certain Genetic Disorders: PGT-A allows for the identification of embryos with specific genetic abnormalities associated with aneuploidy, reducing the risk of having a child with those conditions.
Are there any risks or limitations associated with PGT-A?
- PGT-A may not be suitable for everyone. The risk of abnormal chromosomally abnormal embryos increases with age. While approximately 30% of embryos are abnormal at the age 30, the likelihood of abnormal embryos is 60-70% after 40 3. This means most embryos created from IVF cycles with PGT screening at age 40 will have chromosomal abnormalities.
- It is important to note that PGT is not able to detect all types of genetic abnormalities or guarantee the health of an embryo. While PGT can identify certain chromosomal abnormalities, it does not eliminate the risk of other genetic or developmental disorders.
- False Positives and Negatives: PGT-A, like any genetic testing, may have a small chance of false-positive or false-negative results.
- Embryo Damage: The biopsy procedure carries a minimal risk of damaging the embryo during the removal of cells.
- Limited Testing Scope: PGT-A focuses on assessing the number of chromosomes in embryos, but it does not screen for all genetic conditions. It is not a substitute for comprehensive genetic testing if there is a known family history of specific genetic disorders (cystic fibrosis or muscular dystrophy).
To learn more about our In Vitro Fertilization, or genetic counselling, talk to our experts. Genetic counselling is offered by Igenomix to patients who would like to address questions related to PGS results.
Please visit Igenomix website to schedule your counselling session:
Oasis Fertility Centre, situated in Calgary, Alberta, extends services to Red Deer, Edmonton, and Near By Areas of Calgary serving as a beacon of hope and excellence in reproductive health. Renowned for advanced facilities and empathetic care, the clinic specializes in a comprehensive array of assisted reproductive technologies, including In Vitro Fertilization (IVF).
Our proficient team of fertility specialists, embryologists, and dedicated support staff diligently craft personalized treatment plans, catering to the distinct needs of each patient. Committed to pioneering advancements in reproductive medicine, the clinic empowers countless individuals and couples on their path to parenthood. Through unwavering support, guidance, and top-tier care, Oasis Fertility Centre remains dedicated to transforming dreams of parenthood into reality for its people across these Alberta locations.



